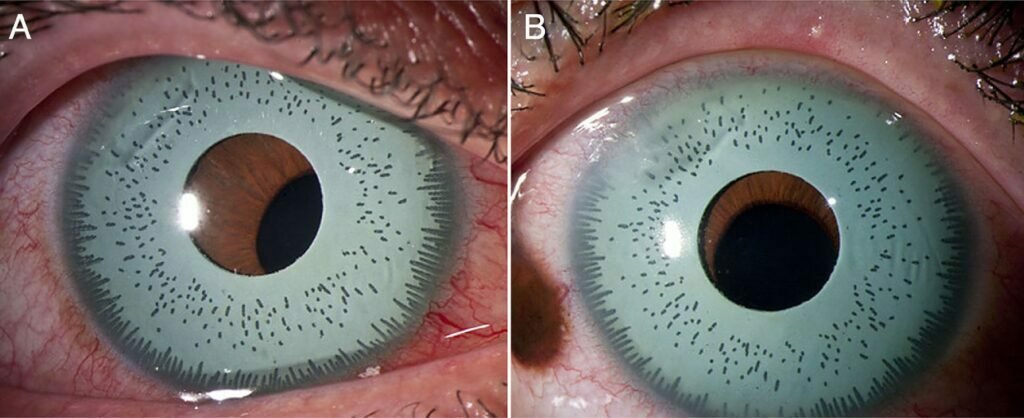
Artificial Iris Implants for Eye Color Change
Important Medical Notice: Artificial iris implants were originally developed for patients with traumatic or congenital iris defects. Their use for purely cosmetic eye color change is not approved by major health authorities due to documented risks, including long-term structural complications. Individuals considering this procedure should undergo a complete ophthalmic evaluation and fully understand the potential consequences.
Artificial iris implants are synthetic devices placed inside the eye to simulate a different iris color. Although the cosmetic effect may appear immediate, the implant alters the eye’s internal anatomy and may influence several important structures including the cornea, drainage angles and lens.
To understand where artificial iris implants fit within the broader category of elective eye color operations, visit:
Eye Color Change Surgery.
For non-incisional medical alternatives, see:
Laser Eye Color Change.
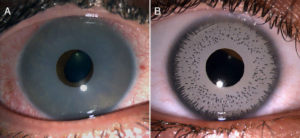
How Artificial Iris Implants Work
The procedure involves inserting a colored synthetic disc into the anterior chamber of the eye. The device sits in front of the natural iris and alters the eye’s appearance by blocking or filtering incoming light. However, the implant’s presence may interfere with delicate internal structures and fluid pathways.
- Intraocular placement: the implant is positioned inside the eye, not on the corneal surface.
- Permanent presence: although removable, risks may persist even after explantation.
- Anatomical impact: may narrow the drainage angle or cause mechanical irritation.
Cosmetic Expectations and Limitations
The color effect produced by an implant may appear uniform but often lacks natural iris patterns or depth. Some patients report glare, reflections, or a flat two-dimensional appearance, especially in strong lighting conditions.
Medical Risks and Complications
Corneal Endothelial Cell Loss
One of the most serious risks is progressive endothelial damage. Because endothelial cells do not regenerate, cell loss may lead to corneal swelling, reduced clarity, or the need for corneal transplantation.
Glaucoma and Elevated Intraocular Pressure
The implant may restrict fluid outflow by narrowing or obstructing the drainage angle. This can cause secondary glaucoma, which may damage the optic nerve and lead to irreversible vision loss without timely management.
Chronic Inflammation (Uveitis)
Prolonged intraocular inflammation is a frequent complication. Persistent inflammation increases the risk of synechiae, cataract formation, and long-term discomfort.
Cataract Formation
Altered fluid dynamics and chronic inflammatory processes may accelerate cataract development, even in younger individuals.
Implant Migration or Mechanical Irritation
If the implant shifts position, it may damage adjacent tissues or worsen visual symptoms.
Vision-Related Symptoms
- Glare and haloes
- Reduced contrast sensitivity
- Photophobia
- Intermittent or persistent blur
Some of these visual symptoms may continue after implant removal.
Regulatory and Safety Status
Cosmetic artificial iris implants are not approved by regulatory authorities for elective eye color change. Published studies and clinical reports consistently document elevated complication risks.
Independent professional guidance:
American Academy of Ophthalmology.
Trauma and Emergency Considerations
In the event of ocular trauma, an artificial iris implant can complicate surgical evaluation and may worsen prognosis by obscuring wounds or increasing operative difficulty.
Implant Removal
Removal is possible but does not guarantee return of baseline vision. Depending on the degree of prior tissue damage, some issues—such as glaucoma, endothelial cell loss or chronic inflammation—may persist.
- Existing endothelial damage may continue to progress
- Glaucoma-related optic nerve damage may be irreversible
- Visual symptoms may not fully recover
Who Should Avoid This Procedure?
Artificial iris implants are not suitable for individuals with:
- Glaucoma or narrow angles
- Low endothelial cell density
- Corneal dystrophies
- Chronic uveitis or inflammatory tendencies
- Any existing ocular pathology
Conclusion
Artificial iris implants can create an immediate cosmetic effect, but they carry significant medical risks including corneal damage, glaucoma, chronic inflammation and potential long-term visual impairment. The procedure is not approved for cosmetic use, and removal may not reverse prior damage. Patients should carefully consult qualified specialists and consider alternative, medically supervised options.
FAQ – Artificial Color Iris Implants
Are artificial iris implants safe for cosmetic eye color change?
Most ophthalmologists consider cosmetic iris implants unsafe. Published complications include glaucoma, corneal endothelial loss, chronic inflammation, and even permanent vision damage. These devices were never designed for healthy eyes seeking cosmetic color change.
Can artificial iris implants be removed if the result is unsatisfactory?
Removal is possible, but it is neither simple nor without risk. In many cases, damage to the cornea or angle structures has already occurred by the time explantation is considered. Removing the implant does not guarantee that vision or corneal health will fully return to the pre-surgery state.
Are there any situations where iris implants are medically justified?
Yes. Certified medical iris prostheses can be used in selected cases with severe traumatic iris defects or congenital abnormalities. These medical indications are very different from placing implants in healthy eyes purely for cosmetic purposes.
Why do artificial iris implants cause so many complications?
Because the implant sits inside the anterior chamber, it disrupts natural fluid flow, touches delicate structures, and interferes with normal iris and corneal function. This increases the risk of high intraocular pressure, endothelial cell loss, inflammation, and long-term vision problems.
Is there a safer approach for cosmetic eye color change?
Non-incisional medical approaches exist, but suitability must be determined through a full ophthalmic examination.